HPTN 096: Building Equity Through Advocacy is an HIV prevention study aiming to reduce HIV rates among Black men (inclusive of cisgender and transgender men) who have sex with men (MSM) in the southern United States.
The study is designed to address structural, social, and behavioral issues that may make accessing healthcare difficult while working to improve existing HIV care and prevention options in communities that have been adversely affected and negligently supported.
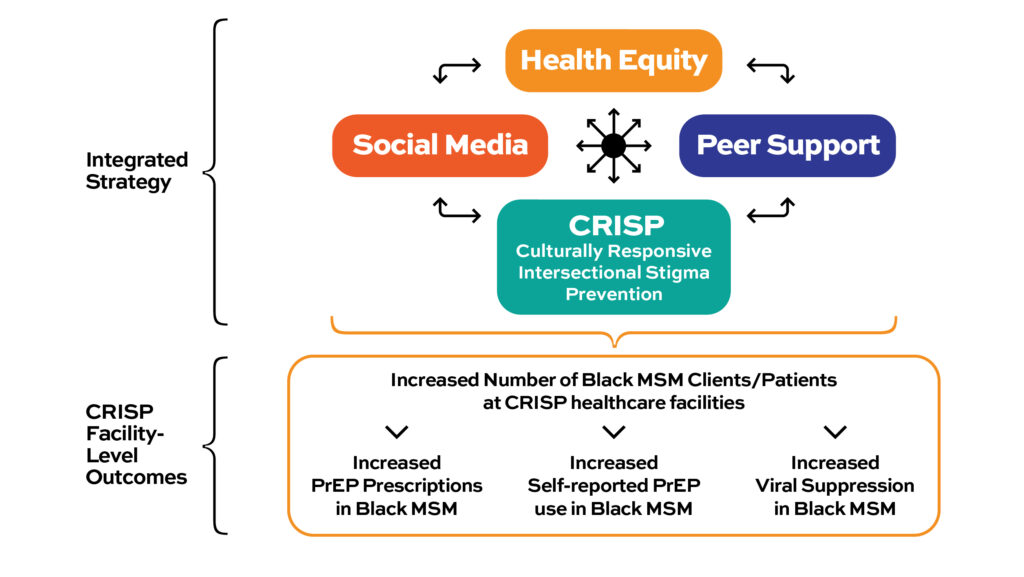
How Does It Work?
The intervention is designed to increase HIV testing in all Black MSM, increase viral suppression among those living with HIV, and increase PrEP use (pre-exposure prophylaxis) use among those living without HIV. The study strategy will be implemented in five (5) communities in the southern U.S. and the effects of the strategy will be measured at healthcare facilities participating in the study.
Details of each study component include:
Peer Support
Serving as role models, trained Peer Supporters establish mutually supportive relationships to exchange knowledge, connect clients with resources, and provide education on HIV-related topics.
Health Equity
Partnering with local community-based organizations to leverage or build coalitions focused on advocacy strategies to address social and structural barriers to HIV prevention and care for Black MSM in the southern U.S.
CRISP
The Culturally Responsive Intersectional Stigma Prevention (CRISP) program is a package of training, skills building, technical assistance, and quality improvement activities designed to help healthcare facilities optimize the experience of Black MSM along the HIV continuum of prevention and care.
Social Media
Leveraging digital tools and social media best practices to provide HIV-related information to Black MSM in the southern U.S. by promoting healthy behavior change and increasing awareness of study activities.
GET INVOLVED
HPTN 096 was developed and built upon a community-centered approach. Each component will involve tailored community engagement activities for the purposes of gaining participation in, awareness of, or acceptability for each part of the study. These community engagement activities are integrated into each component of the intervention and include the formation of a Community Advisory & Strategies Group, component-specific engagement, and indirect recruitment for the cross-sectional assessment.
Frequently Asked Questions
Why is the HIV Prevention Trials Network (HPTN) conducting this study?
Black MSM living in the southern U.S. are among the hardest hit by the HIV epidemic. In 2019, MSM accounted for 60% of the new HIV diagnoses among African Americans in the South – nearly twice that of their other racial/ethnic counterparts. Homophobia, femmephobia, and other related stigmas, discrimination, and inconsistent or challenging access to adequate healthcare, are all factors in the continued epidemic.
Despite the advances in effective biomedical interventions such as oral or injectable PrEP, the reach and impact of these interventions are lacking in the Black MSM community. HPTN 096 is designed to address structural, social, and behavioral issues that may make accessing health care difficult while working to improve existing HIV care and prevention options in communities that have been adversely affected and negligently supported.
To learn more about the HIV Prevention Trials Network, click here.
How is the study trying to reduce HIV?
HPTN 096 is testing an integrated strategy intervention to reduce HIV among Black MSM living in the southern US. The intervention is designed to increase HIV testing in all Black MSM, increase viral suppression among those living with HIV, and increase pre-exposure prophylaxis (PrEP) use among those living without HIV.
The intervention is made up of four components:
- Health Equity
- Peer Support
- Social Media
- Culturally Responsive Intersectional Stigma Prevention (CRISP)
Where is the study taking place?
Five (5) communities are participating in HPTN 096. They include:
- Dallas, TX
- Montgomery, AL
- Fort Lauderdale, FL
- Memphis, TN
- Atlanta, GA
The study strategy was piloted in 2 communities (Dallas, TX, and Montgomery, AL) in 2022. In 2023, the study design is being refined to incorporate lessons learned from the pilot, and it is anticipated that full study roll-out to all five study communities will begin in 2024.
Click here to learn more activities and resources in each study community.
How will we know if the HPTN 096 study intervention works?
The main study outcomes will be measured by looking at electronic medical record data from participating healthcare facilities in the CRISP component to see if there are changes from before to after the study in three main areas: viral suppression, PrEP uptake, and the number of Black MSM who receive services at these facilities.
Other study outcomes will look at the implementation and potential impacts of each component of the strategy.
A sample of Black MSM will be recruited from participating healthcare facilities to complete surveys.
What is the timeline of the study?
The study strategy was piloted in 2 communities (Dallas, TX, and Montgomery, AL) in 2022. In 2023, the study design is being refined to incorporate lessons learned from the pilot, and it is anticipated that full study roll-out to all five study communities will begin in 2024.
Study implementation will last approximately 2 years and is expected to be completed by 2027.
